
- No Results
- Global
-
Australia

-
Austria

-
Azerbaijan

-
Brazil

-
Belgium

-
Canada

-
Chile

-
China

-
Costa Rica

-
Croatia

-
Czech Republic

-
Denmark

-
ESTONIA

-
Finland

-
France

-
Germany

-
Hong Kong

-
Hungary

-
India

-
Italy

-
Ireland

-
Japan

-
Korea

-
Latvia

-
Lithuania

-
Malaysia

-
Mexico

-
Morocco

-
Netherlands

-
New Zealand

-
Norway

-
Philippines

-
Poland

-
Portugal

-
Romania

-
Singapore

-
Slovakia

-
Slovenia

-
Spain

-
Sweden

-
Switzerland

-
Taiwan

-
Turkey

-
United Kingdom

-
UNITED ARAB EMIRATES

-
United States

-
Vietnam

In vitro Cytotoxicity Assay: Agar Diffusion Test (mouse cell line L929)
Contact usIn the Agar Diffusion Test the test material is analysed for its leachable cytotoxic contents after diffusion through an agarose layer.
The test system is used to simulate penetration of leachable substances released from the test item into the tissue of the patient through a barrier (e.g. skin). This test is applicable if those leachable substances can diffuse through, but do not react with the agar layer.
As the agarose layer is stained with neutral red, the cytotoxic potential of the test material is determined via grading of the decolorisation area and assessment of cell lysis of the cell culture.
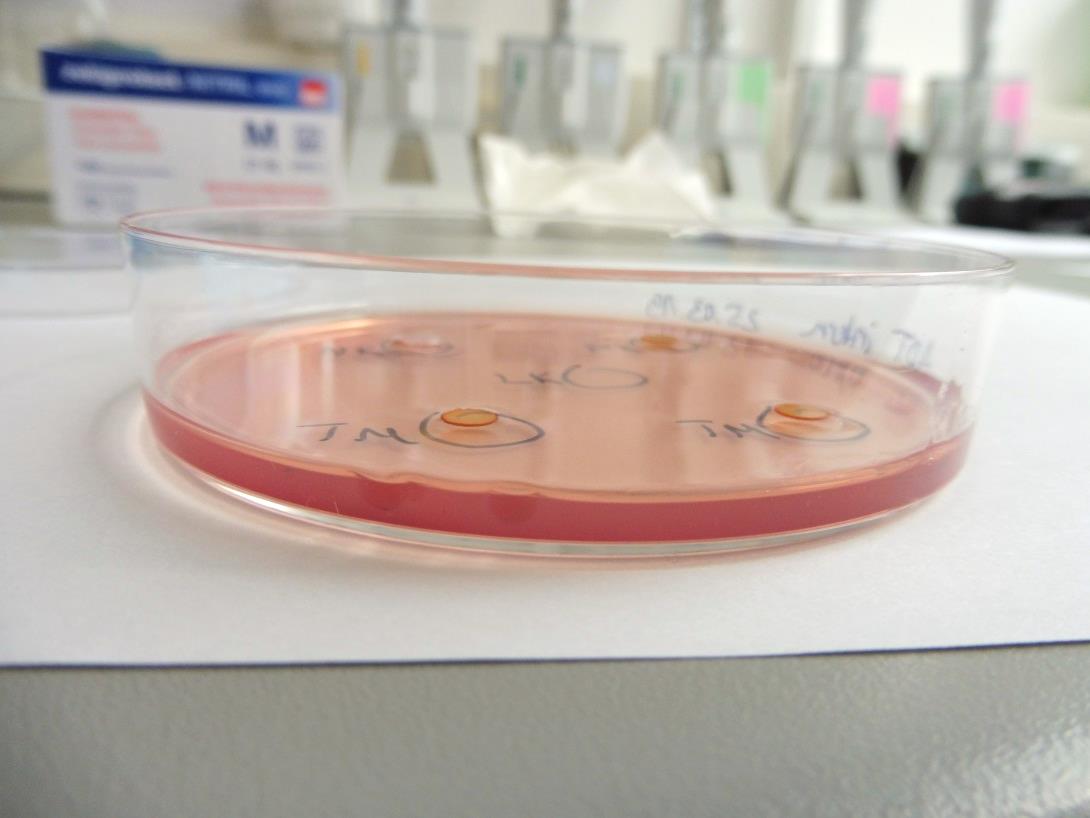
Assessment of Cytotoxicity by Microscopic Evaluation after Indirect Cell Contact
- The subconfluent cell monolayer is covered with an agarose layer and stained with neutral red.
- The test item or an extract of the test item are applied on the agarose layer in indirect contact to the cells.
- Cytotoxicity is determined microscopically after an incubation period of 24 h. Therefore, the decolorisation index and the lysis index are determined qualitatively (see Table).
Protocol |
|
|
Cell line |
L929 cells (ATCC No. CCL1, NCTC clone 929 (connective tissue mouse), clone of strain L (DSMZ)) |
|
Analysis |
Microscopic evaluation |
|
Incubation time |
24 h at 37 ± 1°C |
|
Quality controls |
Solvent Control: DMEM 10% FBS Negative control: High-density polyethylene material Positive control: 0.1% Zinc-diethyldithiocarbamate (ZDEC) (Hatano Research Institute) |
|
Data delivery |
Cytotoxicity according to grading scores |
|
Positive prediction |
Cell reaction is interpreted according to ISO 7405 or ISO 10993-5 |
References
- ISO 7405: 2018, “Dentistry - Evaluation of biocompatibility of medical devices used in dentistry - Test procedures specific to dental materials“
- ISO 10993-5: 2009 “Tests for in vitro cytotoxicity”

