Pharmaceutical Gas Testing


The contamination of classified rooms is a real challenge for the pharmaceutical industry. The fluids used for the production (water, clean steam and vapor of gas) are a potential source of product contamination and crosscontamination from one room to another. The quality of the fluids used should be under control in order to guarantee the quality of the product manufactured on site.
The quality of the pharmaceutical water (purified water or water for injection) is mastered for many years by the pharmaceutical industry and undergoes specific qualification and monitoring plans. Based on this experience, the authorities are focusing now on the quality of pharmaceutical gases. Indeed, gases are used during the production process as an excipient or "invisible helper" in contact of the products such as inerting agent.
Gases that are commonly used in the pharmaceutical industry are:
- Nitrogen for inerting or flushing
- Air for flushing
- Oxygen for fermentation
- Carbon dioxide for extraction and purification
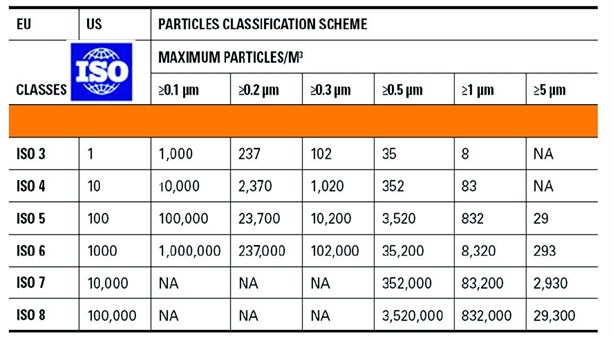
The quality of those gases is specified in the European and U.S. pharmacopeias.
Performing the testing of those gases is a real challenge for the industry since it requires:
- Dedicated analytical equipment according to the pharmacopeias with the appropriate qualification
- Dedicated sampling method fully validated
- Trained and qualified technician with special focus on safety and security issues
In addition, the quality evaluation should be performed at the use point and means access to classified areas.
Besides the pharmacopeias specifications, the gas should also be tested in terms of particular contamination and bio contamination. The contamination of the gas should not be higher than the contamination of the room where it is used. As an example, a gas used in an ISO 5 room, corresponding to the class 100, should be analysed according to the ISO 5 specification in terms of particles contamination.
The specifications for the particular contamination and the bio contamination are reported in tables 1 and 2 on the reverse.
Eurofins BioPharma Product Testing's network of laboratories have developed the full set of methods with all the dedicated equipment to perform all the tests required for the evaluation of the quality of the gases and the qualification of the pipe works for the pharmaceutical industry.
Table 1: Specification and methods according to the European Pharmacopeia
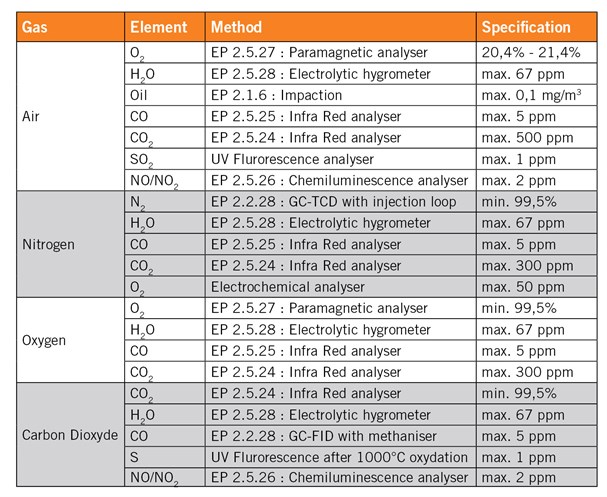
Table 2: ISO Clean Room Standard for Particles