Bevacizumab Biosimilar Assays

A diverse set of methods to evaluate comparability of both the biosimilar drug and it's clinical performance
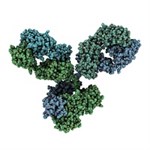
Bevacizumab (Avastin®) is a recombinant humanized monoclonal antibody that inhibits angiogenesis by binding to the vascular endothelial growth factor A (VEGF-A). Bevacizumab contains human IgG1 framework regions (93%) and the complementarity-determining regions (CDR) of a murine antibody (7%) that binds to VEGF. Bevacizumab binds to VEGF extracellularly to prevent interaction with VEGF receptors (VEGFR) on the surface of endothelial cells, thereby inhibiting stimulation of new blood vessel formation.
Bevacizumab is currently licensed to treat various cancers, including colorectal, lung, breast (outside the USA), glioblastoma (USA only), kidney and ovarian
Evaluation of the comparability of bevacizumab biosimilars to the innovator drug should follow the guidelines laid out by the FDA and EMA. The analysis should be multifactorial, taking into account both the physicochemical characteristics and clinical performance of the biosimilar compared to the innovator. Eurofins Bioanalytical Services offers a range of off-the-shelf bevacizumab assays for comparability testing of biosimilars including:
Clinical Assays
 |
Bevacizumab PK Assay
|
 |
Bevacizumab ADA
|
Drug Assays
 |
Fc Receptor Binding
|
|
|
Representative standard curve for detection of bevacizumab in human serum. |
Contact Us for more information


















































