Accredited dietary and food supplements GMP process certification to 21 CFR 111/117 and NSF/ANSI 455-2-2024

Dietary and food supplements exported to the USA or sold within the USA must comply with Good Manufacturing Practices (GMPs) as set forth in by US federal regulations 21 CFR part 111 and applicable portions of 21 CFR 117, regardless of where in the world they are made.
To support supplements manufacturers and brands demonstrate regulatory compliance, a commitment to best practices and quality, and meet increasingly stringent US retailer requirements, Eurofins Assurance offers dietary and food supplements GMP process certification program covering these important regulations and standards in the US market, namely 21 CFR 111/117 and NSF/ANSI 455-2-2024. This programme is accredited to ISO/IEC 17065 by ANAB.
Certification requirements of 21 CFR 111/117 and NSF/ANSI 455-2-2024
Working in compliance with ISO 17065 standards for impartial conformity assessment, our dietary and food supplements GMP process certification program provides a smart solution for industry to stay compliant cost-effectively. Companies can choose between two certification technical scopes to best meet the requirements specified by customers and relevant stakeholders. Both scopes offer a process certification approach based on international standards for conformity assessment. This approach is designed to evaluate whether dietary supplement GMPs are fully implemented across all product types and major manufacturing technologies used onsite. While both options follow the process certification approach, the technical standards evaluated vary depending upon the scope selected:
Audit scope of 21 CFR 111/117 includes21 CFR 111, 21 CFR Part 121, 21 CFR Part 117 (Subparts A, B, F), and 21 CFR Part 1.511 and is organized following the regulations:
- Quality System
- Facilities and Equipment
- Material and Supplier Management
- Production System
- Packaging System
- Laboratory System
Audit scope of NSF/ANSI 455-2-2024 includes 21 CFR part 111, 21 CFR Part 117, 21 CFR part 121, 21 CFR Part 11, 21 CFR Part 1.511, 21 CFR Part 1.906, 21 CFR Part 1, Subpart O and 21 CFR Part 1.908. The standard is organized in a quality systems approach similar to ISO 9001 and includes:
- Context of the Organization
- Leadership
- Planning
- Support
- Operation
- Performance Evaluation
- Improvement
- Non-Grading Requirements
A GMP certificate of conformity and the Eurofins Assurance certification marks are issued to the company after all program requirements are met and the company is able to market its certification its customers and distinguish itself from competitors.
Dietary supplement GMP certification process overview
To initiate the certification process, Eurofins will collect detailed information about your company manufacturing sites, including some pre audit documentation to ensure preparedness and proper audit scope and duration is agreed.
The audit duration is based upon the scope and complexity of the operation but is usually 2-3 days for a single location.





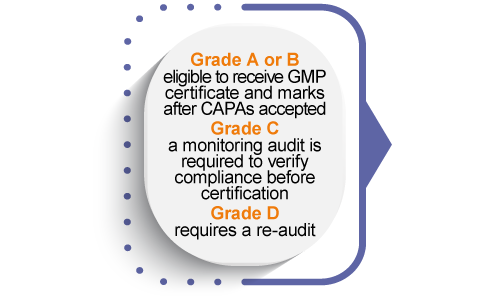


Dietary Supplements GMP process certification documentation
Manufacturers who meet all certification requirements receive a GMP Certificate of Conformity issued by Eurofins Healthcare Assurance. Our dietary supplement GMP Certificates are accepted by some renowned retailers and brands as conformity document.
Companies are also licensed to use and display the Eurofins Dietary Supplement GMP Certification Mark on corporate marketing materials to communicate the achievement and highlight the company commitment to quality and continuous improvement.
Please refer to the Policies and Procedures for Good Manufacturing Practices (GMPs) and Management Systems Certification. The certification is conducted by Eurofins Assurance Audit and Certification Services US, LLC and the Terms & Conditions can be viewed here.


Why choosing Eurofins for your dietary supplement GMP certification
With experts in the US and around the world, the Eurofins Healthcare Assurance team have decades of experience with dietary and food supplements regulations and manufacturing best practices to support your FDA compliance and meet retailer and brand expectations. Our team are third party conformity assessment experts and leverage the most experienced, well-trained auditors located globally to support customers with geographic proximity.
For dietary supplements GMP certification, Eurofins Assurance also offers:

This programme is accredited to ISO/IEC 17065 by ANAB, demonstrating competence, impartiality, and credibility. You can view relevant scope of accreditation from the ANSI National Accreditation Board (ANAB) here.
Our certification activities are provided by independent Certification Bodies separately from any consulting activities. Impartiality is safeguarded by Eurofins Assurance’s relevant policies to avoid conflicts of interest.




© Eurofins Assurance 2025 Personal data protection policy

















































